crystalline solids and amorphous solids
In part of the older literature, the term has been used synonymously with glass. Nowadays, "amorphous solid" is considered to be the overarching concept, and "glass" the more special case: A glass is an amorphous solid that transforms into a liquid upon heating through the glass transition.

Crystalline Solids
It is difficult to make a distinction between truly amorphous solids and crystalline solids if the size of the crystals are very small. Even amorphous materials have some short-range order at the atomic length scale due to the nature of chemical bonding. Furthermore, in very small crystals a large fraction of the atoms are located at or near the surface of the crystal; relaxation of the surface and interfacial effects distort the atomic positions, decreasing the structural order. Even the most advanced structural characterization techniques, such as x-ray diffraction and transmission electron microscopy, have difficulty in distinguishing between amorphous and crystalline structures on these length scales.

Picture 4, -, Amorphous solid
Amorphous phases are important constituents of thin films, which are solid layers of a few nm to some tens of µm thickness deposited upon an underlying substrate. So-called structure zone models were developed to describe the microstructure and ceramics of thin films as a function of the homologous temperature Th that is the ratio of deposition temperature over melting temperature. According to these models, a necessary (but not sufficient) condition for the occurrence of amorphous phases is that Th has to be smaller than 0.3, that is the deposition temperature must be below 30% of the melting temperature. For higher values, the surface diffusion of deposited atomic species would allow for the formation of crystallites with long range atomic order.

When amorphous solids

over crystalline solids.

amorphous solid: atomic

A Crystal is a solid formed by
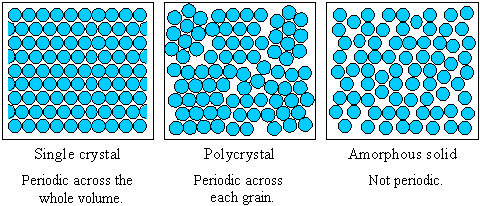
and amorphous solids.

crystalline solid (right).

Solids: crystalline vs.

Solids are divided into

Crystalline Solids
It is difficult to make a distinction between truly amorphous solids and crystalline solids if the size of the crystals are very small. Even amorphous materials have some short-range order at the atomic length scale due to the nature of chemical bonding. Furthermore, in very small crystals a large fraction of the atoms are located at or near the surface of the crystal; relaxation of the surface and interfacial effects distort the atomic positions, decreasing the structural order. Even the most advanced structural characterization techniques, such as x-ray diffraction and transmission electron microscopy, have difficulty in distinguishing between amorphous and crystalline structures on these length scales.

Picture 4, -, Amorphous solid
Amorphous phases are important constituents of thin films, which are solid layers of a few nm to some tens of µm thickness deposited upon an underlying substrate. So-called structure zone models were developed to describe the microstructure and ceramics of thin films as a function of the homologous temperature Th that is the ratio of deposition temperature over melting temperature. According to these models, a necessary (but not sufficient) condition for the occurrence of amorphous phases is that Th has to be smaller than 0.3, that is the deposition temperature must be below 30% of the melting temperature. For higher values, the surface diffusion of deposited atomic species would allow for the formation of crystallites with long range atomic order.

When amorphous solids

over crystalline solids.

amorphous solid: atomic

A Crystal is a solid formed by

and amorphous solids.

crystalline solid (right).

Solids: crystalline vs.

Solids are divided into

0 Comments:
Post a Comment
Subscribe to Post Comments [Atom]
<< Home